Food is perishable, so they need certain conditions of treatment, conservation, and handling; its main cause of deterioration is the attack by different types of microorganisms such as bacteria, yeasts, and molds; therefore, there are various techniques for food Preservation these products, with cooling at very low temperatures being one of the most used, thanks to its effectiveness.
Various procedures have been developed to keep food usable, some of which date back many centuries and even millennia. However, its application on an industrial scale began towards the end of the 18th century (Nicolás Appert discovered in 1795 the preservation process in cans by heat sterilization and exclusion of air). At the same time, artificial drying procedures were introduced, which quickly spread to the most diverse foods (fruits, vegetables, milk, eggs, meat, fish, etc.).
At the same time, other methods were developed, such as smoking, salting, and preserving with vinegar, spices, sugar and various chemical products.
It was also known in ancient times that it was possible to considerably prolong the shelf life of food Preservation by keeping it at low temperatures, using natural cold (underground cellars, cold springs, snow, and ice) in the case of certain foods and beverages.
In the same way, refrigerating mixtures (snow with salts and acids) have been used for centuries; but low temperatures could be used industrially in the first half of the 19th century.
The food Preservation of fresh food is one of the first applications of artificial cold. It was soon known that temperatures above 0º C only prolong the shelf life of many foods to a limited extent, which is why in 1860, it was switched to freezing due to the interest associated with the global meat trade.
Unlike other procedures, cold storage is the only method to ensure that the product’s natural taste, smell, and appearance hardly differ from the natural one.
Although canned fruits, smoked fish, salted meat, dried vegetables, jams, etc., can be excellent and tasty foods, they differ greatly from fresh products. In contrast, it can keep cold-preserved or frozen foods for months without alteration if the treatment is correct.
It is worth recognizing that conservation is limited when removed from the cold room, so must consume them quickly.
It is estimated that more than 20% of all food produced in the world is lost due to the action of microorganisms.
In this sense, maintaining the optimal conservation and storage conditions for each food during the time it lasts presupposes the so-called “cold chain”, which includes transportation, wholesale and retail sales, and the consumer.
Refrigeration prevents the growth of microorganisms that can withstand temperatures above 45º C and many that can survive temperatures ranging from 15 to 45º C.
Table of Contents
Factors Affecting Food Preservation Quality during Cold Storage
Certain aspects will intervene in the good food Preservation of the properties of food during its preservation in the cold:
Influence of temperature
Decomposition processes depend largely on temperature and become slighter and slower as temperature decreases.
The “evaporation” of water and the weight loss associated with it decrease with decreasing vapour pressure, which is, in turn, lower the temperature (at 30º C, it is 31.8 mm Hg, and at 0º C, only 4.6 mm Hg.). In the same way, the vapour pressure of the volatile aromatic components decreases.
From the kinetic study of chemical reactions, it is known that the reaction rate of all processes decreases rapidly with decreasing temperature (k = Koe –E / RT). The temperature coefficients of successive processes are not all the same, but on average, it can be accepted that for every 10Âo C that the temperature decreases, the speed of a process becomes 2 or 3 times lower as these reactions mean, in most cases, decreases in the commercial value of food, the duration doubles or triples for every 10º C decrease in temperature. If 2.5 is taken as the average value, we can expect that most foods can be kept at 0°C for more than fifteen times that they can be kept at 30°C.
For some foods, the temperature coefficient of chemical processes increases sharply near the freezing point; thus, fish can be kept at 0º C for a much longer time than at 1º C and at temperatures lower than –1º C for longer than at 0º C. In some fruits, the temperature coefficients of the superimposed reactions are so different that when approaching 0º C, physiological alterations occur in the system, leading to the appearance of the so-called diseases due to cold storage (cold burning).
As far as the growth of microorganisms at different temperatures is concerned, it is known that the different species prefer certain intervals of favourable temperatures. If thermophiles species are ignored, whose multiplication already stops at 45º C, the most favourable zone for cryophilic remains between 15º C and 20º C and for the mesospheric species between 30 and 35º C. The mesospheric species stop multiplying below 10º C, while this occurs for the cryophilic species below –7º C.
Therefore, we can say that microorganisms’ growth is greatly reduced with decreasing temperature in the temperature zone that interests us. However, we should note that many microorganisms do not die even at the lowest temperatures used by this preservation system, so they begin to multiply again as soon as the food reaches higher temperatures again. Can keep cold or frozen foods for months practically without alteration if the treatment is correct
Influence of relative humidity during storage
Together with temperature, relative humidity strongly influences the preservation of cold-stored foods. Evaporative weight loss decreases as the relative humidity of the air increases in the store, being proportional to the difference between the partial pressures of water vapour in the air and on the surface of the stored product. The relative humidity is the ratio between the partial pressure of water vapour and its saturation pressure at a given temperature. Can reduce weight losses by packaging the products.
On the other hand, high relative humidity favors the multiplication of microorganisms, especially at high storage temperatures. Thus, for example, bacteria reproduce slowly at a relative humidity of 75%, but weight losses are high; On the contrary, for relative humidity between 90-95%, there are small weight losses, but the multiplication of bacteria can only be kept within a tolerable limit if the storage temperature is lowered as close to 0º C.
In general, the relative humidity can be higher the lower the temperature. In freezing chambers, the water vapour content in the air and on the surface of the products is very small, so the differences between the partial pressures take very low values. Weight losses per unit of time remain low, although long storage periods must often be expected. Weight losses of frozen meat and fish are halved by making the temperature ten degrees lower.
However, a drying of the surface that worsens the appearance of the products is very effective in reducing the multiplication of microorganisms. Such drying greatly reduces the commercial value of some products since, for example, it is required that the fish retain its shine and its superficial mucus and that the fruits do not have a rough surface.
The shelf life of refrigerated vegetables depends on the variety, partly stored, the conditions of their collection, and the temperature during their transport.

Influence of air circulation Air
The movement also influences the quality and preservation of products subjected to refrigeration, freezing and storage. As far as weight losses are concerned, water evaporation takes place more quickly with air circulation. For the transport of matter, the same laws apply to the transport of heat. So in the refrigeration and freezing processes, the greater loss of substances per unit of time is sufficiently compensated with the shorter refrigeration or freezing time. It is useful; therefore, the use of high air circulation speeds.
Air circulation prevents moisture from rising to the surface of the products and contributes to the rapid formation of a dry surface that offers more unfavorable conditions for the multiplication of bacteria. For this reason, air circulation is preferred in the storage of fresh meat above 0°C (for example, in slaughterhouses), and large daily weight losses in short-term storage are accepted.
Air circulation is also used in cold rooms for eggs, fruits and vegetables to allow a more homogeneous temperature distribution than with still air.
In the prolonged storage of frozen products, the multiplication of bacteria is prevented, and refrigeration with still air is recommended. This is especially the case in rooms for frozen fish, whose appearance is greatly affected by weight loss. In this case, the glazing or packaging of the fish represents an aid in avoiding the loss of water vapour.
Despite the existence of other methods of food preservation, refrigeration is the most widespread and applied in the domestic and commercial sphere.
Refrigeration
Refrigeration consists of preserving products at low temperatures but above their freezing temperature. It can be said that the refrigeration is framed between 1º C and 8º C. In this way, it is achieved that the nutritional value and the characteristics of flavor, texture, and smell hardly differ from those of the products at the beginning of their storage. For this reason, consumers consider fresh refrigerated products as healthy foods.
Refrigeration prevents the growth of microorganisms that can withstand temperatures above 45º C and many that can survive temperatures ranging from 15 to 45º C.
However, to achieve the expected result, other factors must also be taken into account and temperature and other storage conditions. The shelf life of refrigerated vegetables depends on the variety, partly stored, harvest conditions, and temperature during transportation. For processed foods, it depends on the type of food, the intensity of the processing received (mainly on microorganisms and enzymes), and hygiene in the preparation and packaging, among others.
In the case of fruits, the rate of respiration varies with temperature. In fruits with a climacteric pattern (they increase the production of CO2 when they increase their maturation), a sudden increase in their respiratory activity occurs during storage. These fruits include avocado, mango and papaya. Fruits with a non-climacteric pattern do not present the above behaviour: orange, grapefruit, and pineapple. The respiration of vegetables is similar to that of fruits with a non-climacteric pattern.
When the temperature of some fruits and vegetables decreases below a certain value, undesirable changes occur in them, which are known as cold damage, causing adverse reactions in these foods.
In animal tissues, when the supply of oxygenated blood ceases as a result of slaughter, aerobic respiration ceases, and anaerobic respiration begins, whereby glycogen is transformed into lactic acid, causing a decrease in pH initiating a process called rigor. Mortis (is a recognizable sign of death caused by a chemical change in the muscles. Those results in a state of stiffness and inflexibility in the limbs and difficulty in moving or handling the corpse).
As a result of this process, muscle tissue hardens, becoming inextensible. For this process to be carried out and the product to acquire the appropriate color and texture, it must be carried out under refrigeration conditions to stop the development of microorganisms.
Food preservation refrigeration can be applied alone or in combination with other techniques, such as irradiation, modified and controlled atmospheres, and packaging in modified atmospheres.
Refrigeration finds a great application in preparing prepared meals in which cooking-chilling systems are applied.
Refrigeration
Time The determination of the refrigeration time constitutes an element of practical importance. It allows knowing the time necessary for a product to reach a given temperature in its thermal center starting from an initial degree, the temperature of the cooling medium, geometric configuration, type of container, etc.
This result can be used to calculate the product load corresponding to the thermal load.
One way that can be used to determine this time is a graphical method. This is based on graphs for each of the simple geometric shapes, spheres, parallelepipeds, and cylinders, where a temperature factor is related, the Fourier number that links the thermal diffusivity, the size of the product and the cooling time, and the Biot number that links the heat transfer coefficient, the conductivity and the thickness of the product.
The method described above assumes that heat transfer is unidirectional. When the heat transfer takes place in more than one direction, obtaining the time above leads to infinite series, demonstrating the possibility of being limited only to the first of its terms.
Characteristics of water
Water is the most abundant constituent in most foods in its natural state, which is why it plays an essential role in the structure and other characteristics of plant and animal origin products.
This component present in food preservation can be free water or as bound water. The latter may be more or less strongly bound in a complex manner to other constituents. That is why the state of the water present in a food is as important for its stability as its total content since its aptitude for deterioration will depend on it.
Approximately 80% of the total weight of an animal and even more than a plant corresponds to water. Water is the main component of food derived from animals and plants.
Water is a solvent for the many chemical species that can diffuse and react with each other. Water can also diffuse and participate in various reactions, especially hydrolysis.
The introduction into the water of different chemical species in solution or colloidal suspension gives rise to the so-called colligative properties, which depend on the number of molecules present. In this sense, the decrease in vapor pressure, elevation of the boiling point, decrease in the freezing point, decrease in surface tension, increase in viscosity, and osmotic pressure gradients through semi-permeable membranes, among others, can be mentioned. These properties determine the behavior of food.
Water molecules in the solid-state are linked by hydrogen bonds, which gives rise to the formation of polymers with a crystalline structure in which each molecule is linked to four others.
Different agents influence the structure of water differently. Thus, electrolytes such as Na+, K+, and Cl-, strongly hydrated in solution, decrease the number of hydrogen bonds between water molecules.
Substances in a solution capable of hydrogen bonding by themselves can modify the association between water molecules according to their geometric compatibility with the existing network.
The water, in turn, modifies properties such as the structure, diffusion, reactivity, etc., of the substances in the solution.
Water activity (aw) is a measure of the greater or lesser availability of water in various foods, which is defined by the decrease in the partial pressure of water vapor:
aw = PW / PO
Where “PW” is the partial pressure of water vapor in the food and “PO” is the vapor pressure of pure water at the same temperature.
The water activity (aw) is a relative measure concerning a standard state taken as a comparison. The chosen normal state is that of pure water at which its activity is equal to unity, so food activity is always less than unity. This is because the chemical species present reduces the water’s vaporization capacity.
The main effect that freezing has on food is the damage caused to the cells by the growth of ice crystals.
Freezing
This application of low temperatures is distinguished by the fact that the temperature of the food is reduced below its freezing point. A high fraction of its water changes its physical state, forming ice crystals. This immobilization of the water in the form of ice and the increase in the concentration of the solutes in the unfrozen water causes the reduction of the water activity of the food. Therefore, the preservation of food by this route is the consequence of the combined action of low temperatures and the decrease in its water activity.
Not all the water present in the food can separate as crystals due to freezing. There is a fraction of non-freezable water in the food that corresponds to very low activity (up to 0.3). This water, which is strongly bound to molecular structures, is called bound water, remaining unfrozen at –30º C. It is considered that this water forms a monomolecular layer fixed to polar groups such as NH3 and COO -de proteins and HO- groups of starches, among others. Bound water represents between 5 and 10 percent of the total mass of water contained in the food.
The water in this layer is very difficult to extract and is not available to act as a solvent or reagent.
On the other hand, free or unbound water represents the majority of the water contained in food. However, this water does not spontaneously leave the tissues. This water is found in the form of gels both inside the cell and in the intercellular spaces, its retention being influenced by the pH and ionic forces.
During freezing, water is removed from its normal position within the tissues and converted to ice. This process is partially reversed during thawing, leading to exudate formation. The increase in the concentration of cellular contents can generate undesirable processes in the products.
In freezing chambers, the water vapor content in the air and on the surface of the products is very small, so the differences between the partial pressures take very low values.
Freezing curve
The freezing process in food preservation is more complex than freezing pure water. Because foods contain other dissolved solutes and water, they exhibit freezing behavior similar to solutions.
The temperature evolution with time during the freezing process is called the freezing curve. The typical freezing curve of a solution is shown in the figure below.
This curve has the following sections:
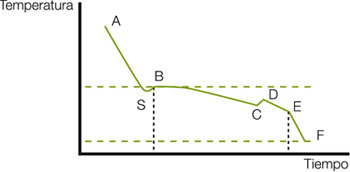
AS: the food cools below its freezing point of less than 0º C. At point S, which corresponds to a temperature below the freezing point, the water remains liquid. This subcooling can be up to 10º C below the freezing point.
SB: the temperature increases rapidly until it reaches the freezing point because when ice crystals form, the latent heat of freezing is released at a rate higher than that extracted from the food.
BC: the heat is eliminated at the same rate as in the previous phases, eliminating the latent heat with ice formation while the temperature remains practically constant. The increase in solute concentration in the unfrozen water fraction causes the freezing point to drop, so the temperature decreases slightly. Most of the ice is formed in this phase.
CD: one of the solutes reaches super-saturation and crystallizes. The release of the corresponding latent causes the temperature to rise to the eutectic temperature of the solute.
DE: crystallization of water and solutes continues.
EF: the temperature of the ice-water mixture drops.
In reality, the freezing curve of foods is somewhat different from that of simple solutions. This differentiation is more marked to the extent that the speed at which freezing occurs is greater.
Freezing
Time The knowledge of the freezing time is a factor of great importance for the design of the process. This time is necessary data to determine the refrigeration speed required for the capacity of the freezing system.
Freezing time prediction can be based on numerical methods and approximate methods. The former is based on the solution of the general energy differential equation. The second, also called analytically, considers simplifications in the differential equation solution.
The first proposed approximate solution corresponds to Plank’sPlank’s equation, which considers a series of assumptions. Despite its limitations, this equation has been widely used, and many of the equations developed later are based on the introduction of modifications to it.
During freezing, water is removed from its normal position within the tissues and converted to ice.
Food Preservation, modifications during freezing
Freezing causes an increase in the concentration of the solutes present. Despite the decrease in temperature, the rate of reactions increases, despite the decrease in temperature by the law of mass action. This increase in the rate of reactions occurs between –5ºC and –15ºC.
This increase in solute concentration causes changes in viscosity, pH, the redox potential of the unfrozen liquid, ionic strength, osmotic pressure, and surface tension, among others. The action of these factors associated with the disappearance of a part of the liquid water causes unfavorable changes in the food, an example of which is the aggregation of proteins. These effects can be limited when the passage through the temperature above range is carried out quickly. This range is called the danger zone or critical zone.
As the volume of ice is greater than that of liquid water, food freezing causes expansion. This dilatation can vary in correspondence with the water content, the cellular disposition, the concentration of solutes, and the freezing medium’s temperature.
These variations that originate in the volume cause internal tensions of great magnitude on the tissues, which can cause internal tears (and even the complete rupture of the plant tissues), which causes loss of liquid during thawing.
Rigour Mortis is a recognizable sign of death caused by a chemical change in the muscles that results in stiffness and inflexibility in the limbs and difficulty in moving or handling the corpse.
The main effect that freezing has on food is the damage caused to cells by the growth of ice crystals. When the freezing rate is slow, ice crystals grow in the extracellular spaces, distorting and breaking the cell walls that contact them. The vapor pressure of the ice crystals is lower than that inside the cells, which causes progressive dehydration of the cells by osmosis and thickening of the ice crystals.
In this way, large ice crystals and increased extracellular spaces originate. Plasmolyzed cells considerably decrease in size. This cellular dehydration decreases the chances of intracellular nucleation. The rupture of the cell walls results from the mechanical action of the large ice crystals and excessive shrinkage of the cells.
During thawing, the cells cannot recover their original shape and fleshiness, the food softens, and the cellular material drips off. The expulsion of part of the cellular content can cause contact between enzymes and their substrates, sometimes found in separate compartments. This is the case, for example, of polyphenol oxidase and polyphenols in foods not previously blanched, which causes an acceleration of enzymatic browning (oxidation reaction in which molecular oxygen intervenes as a substrate, catalyzed by a type of enzyme) during thawing and even during storage.
